Why Add the Acid to the Water?
Hot Grease and Water
You guys may have noticed that you should never drop water into hot grease. But dropping hot grease into water isn't nearly as dangerous, even though it's still not a good idea.
The reason for this is that water boils at 212 degrees fahrenheit. When you drop water into grease that is many times at 400 degrees, it instantly evaporates. When it evaporates it expands. The quicker it does so, the more like an explosion it will be. When the explosion takes place, it will throw hot grease all over the place - including your skin and eyes.
But what about the other way around? I've fried bacon and had a bunch of grease left over and dumped it into water. I've noticed that the explosions are smaller because the grease cools down faster. There's less grease too, which means less heat overall in the system. If you do manage to create an explosion doing this, it will blow water all over the place. Not that bad.

Acid to the Water!
Why add the acid to the water? Because the same general concept described above applies when you're mixing an acid or a base to water. A base is the opposite of an acid and they are corrosive for different reasons. You can usually tell dilute mixtures of the two apart by their taste - acids are sour while bases are bitter. THIS IS AN UNSAFE TEST. WHEN YOU DIE OF POISONING, DON'T BLAME ME! Acids will also cause baking soda to foam while bases won't.
Anyway, enough with the other information and on to the topic at hand. Dissolving strong acids and bases is highly exothermic. In English, this means that mixing water to acids and bases will cause them to heat up a lot. But there's a trick to it. You see, if you add the water to a really strong acid, that water can potentially heat up to the point of boiling. If it does this quickly enough, it will expand and blow the acid out. If it's 16-molar sulfuric acid, it will eat through your clothes before you can wash it off. Most people don't know they've got sulfuric acid on them until it burns through their top layers of skin and starts to sting a bit. Hydrofluoric acid is rare but is an anaesthetic and will numb your skin as it burns the shit out of your skin. I've got a bunch of great and useless information about acids, but that's for later.
So the reason you add the base or the acid to the water is because you're transfering that heat to a greater amount of water - which usually won't result in any miniature explosions. Even if it does, you'll be throwing water all over the place as opposed to acid. So remember: acid to the water!
What the Hell is a Base?
Many people have never heard the word "base" used in the context I'm using it in. There are different types of bases. It can loosely be defined as the opposite of an acid. There are multiple definitions.
Definition number one is anything that has hydroxide in it, or that creates a hydroxide when added to water. Sodium hydroxide is incredibly common (aka lye). Potassium hydroxide (similar to lye). Ammonia is a bit different - it can act like a base on its own for reasons explained in definition two or because when you add it to water it pulls a hydrogen off of a water molecule leaving you with a hydroxide - making ammonium hydroxide. Just so you know, for the chemical you can buy at the store called ammonia, ammonium hydroxide is the exact same chemical with a different name. They're not trying to be deceitful, it's just what some people call it.
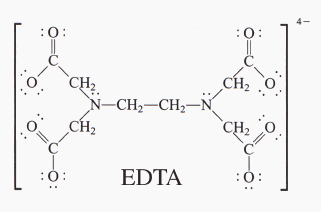
Definition number two is any chemical that donates a pair of electrons without actually giving up permanently that pair of electrons. Hydroxide falls in this category as it has three pairs of electrons to donate. Ammonia also falls in this category as it has one pair it can donate. Sodium carbonate is a weak base, but it still falls in the definition. EDTA - an acronym for a chemical that has a ton of electrons to donate. What happens when these chemicals react with say, a metal, the metal actually has addresses for electrons that aren't filled yet. The base comes in and puts its electrons in those addresses. The metal grabs on to the electrons, but can't actually bind. This is advantageous if you're trying to protect the surface of a metal that won't dissolve with the particular base you're using. It's also advantageous to dissolve the metal if it does so with the particular base you're using. This kind of electron-pair donation is incredibly vital with stabilizing chemicals in the body - hemoglobin for one. Anything that has a metabolic metal in it has bases stabilizing its position and reactivity. Carbon monoxide is a very dangerous base as it donates its electrons to metals incredibly easily and will bind to the iron in your hemoglobin and won't let oxygen bind - essentially suffocating you from the inside. Cyanide is a base that attacks other metabolic metals.
All in all, I like acids more than bases. Sometimes you get a chemical that can act like both. Take EDTA for example. It's got a lot of pairs of electrons to donate, making it a great base. However, those electrons have negative charge that need stabilized by a positive charge. If that positive charge comes from a hydrogen ion, that makes EDTA both an acid and a base at the exact same time. One definition of an acid is a positive-hydrogen-ion chemical. Weird having acids and bases being the same thing, eh? It takes a moment to wrap your mind around.